Automated CPR Devices Market is growing rapidly as Royal Philips receives the U.S. FDA premarket approval for its HeartStart FR3 and HeartStart FRx automated external defibrillators (AEDs)
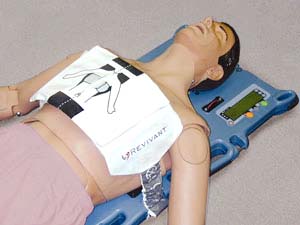
Many new developments in the field of cardiopulmonary resuscitation (CPR) have led to the development of many advanced medical resuscitation technologies. Cardiopulmonary resuscitation (CPR) is a lifesaving technique used in numerous emergencies including heart attack, coronary artery disease (CAD), and heart valve disease. According to the Centers for Disease Control and Prevention (CDC), in 2017, around 365,914 people die due to CAD with around 18.2 million adults in the U.S. of age 20 years and above have CAD. Such massive incidence of heart diseases has increased the demand for appropriate medical devices. Thus, these factors can stimulate growth of the automated CPR devices market. Automated CPR devices assist to deliver high frequency and consistent compressions during the cardiopulmonary resuscitation phase. This is critical to achieving maximum outcomes and reducing the chances of the victim losing consciousness as a result of ventricular tachycardia, ventricular fibril...
